What is EDI in Water Treatment and How Does EDI Water System Work
Table of Contents
Electric Deionization, abbreviated as EDI, belongs to the high-tech green environmental protection technology. Its emergence is a landmark revolution in the water treatment industry, marking the comprehensive water treatment industry into the era of green products. EDI water treatment equipment has the advantages of continuous effluent, no acid-alkali regeneration, and unattended operation. It has gradually replaced the traditional mixed bed as the delicate treatment equipment in preparing pure water systems. This advanced technology has good environmental characteristics and is easy to operate and use. As a result, people increasingly recognize it, and it is widely promoted in pharmaceutical, electronic, power, chemical, and other industries.
What is EDI (electro-deionization) in water treatment?
Electro deionization (EDI) or Continuous Electro deionization (CDI) is a technology that uses ion exchange resins and ion exchange membranes to continuously remove ions and constantly regenerate the resins under the action of a direct current electric field.
Electro-deionization technology is a new process of pure water preparation that combines ion exchange technology, ion membrane separation technology, and ion electromigration technology. It not only uses ion exchange for deep desalination to overcome the incomplete desalination caused by polarization in the electrodialysis process but also uses electrodialysis polarization and water ionization to produce H+ and OH– to achieve continuous regeneration of the resin, avoiding environmental pollution caused by acid and alkali regeneration after ion exchange resin failure. It is the world’s most advanced high-purity water production technology today.
What are the advantages of EDI (electro-deionization) technology?
The EDI process system replaces the traditional DI mixed resin bed to produce deionized water, and its superiority is as follows.
- The organic combination of electrodialysis and ion exchange technology retains the advantages of continuous desalination by electrodialysis and deep desalination by ion exchange.
- Less ion exchange resin consumption than ordinary ion exchange resin column saves more than 95% of the resin.
- Ion exchange resin does not need acid and alkali chemical regeneration, saving a lot of acid and alkali and cleaning water, significantly reducing labor intensity.
- Continuous operation, no waste acid, and alkali discharge. It is clean production technology and green environmental protection products.
- The process is easy to achieve automatic control. Combined with reverse osmosis (RO), ultrafiltration (UF), and other water treatment technology, EDI can form a perfect high-purity water production line.
- The compact structure of the equipment occupies little space, is easy to operate, has a low failure rate, and has no need for repeated settings such as ion exchange beds, one set in use, and one set of regeneration.
- High efficiency of pure water production, high quality of product water, resistivity up to 15~18MΩ▪ cm (25ºC).
Different separation membranes can only be assembled into membrane modules and installed with pumps, filters, valves, instruments, and piping to complete the separation task. A membrane module is a device in which membranes are assembled in some form in a basic unit of equipment that can separate the components of a mixture under specific driving forces. The larger the membrane area, the greater the permeability per unit time. Therefore, when using membrane separation technology in practice, it is necessary to develop membrane modules with the largest membrane area per unit volume.
How does the EDI (electro-deionization) water system work?
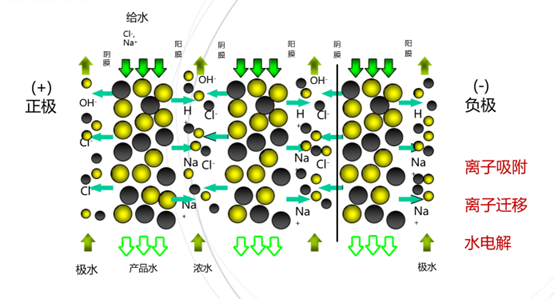
An EDI stack consists of several units connected in parallel. An EDI unit consists of an ion exchange resin, an ion exchange membrane, and a DC electric field. The ion exchange membrane is divided into anion exchange and cation exchange membranes. The cation exchange membrane allows only cations to pass; anions and water cannot pass, while the anion exchange membrane allows only anions to pass. The anion exchange membrane and cation exchange membrane are arranged at intervals to form an alternating concentrated water chamber and fresh water chamber, and the mixed anion and cation exchange resin is filled into the freshwater chamber. Under the action of the applied DC electric field, the cations move toward the cathode, and the anions move toward the anode and enter the adjacent concentrated water chamber through the ion exchange membrane, respectively, thereby reducing the ion content in the freshwater channel water and achieving the purpose of water purification.
There are two main functions of ion exchange resin: ① In the freshwater chamber, the conductivity of the ion exchange resin is 2~3 orders of magnitude higher than the water in contact with it. Therefore, the ion migration in the freshwater chamber is done through the ion exchange resin. This process lowers the resistance of the water production channel, enhances the ion migration, and improves the electric deionization capacity, thus improving the water quality of the product water. ② During operation, concentration polarization occurs in the diffusion layer at the interface between the resin and the water phase contact. A high potential gradient is established when the polarization develops to a certain degree, forcing the water to dissociate into H+ and OH-. This dissociation of H+ and OH-, in addition to participating in the load current, also allows the resin to be in an electrical regeneration state, thus completing the regeneration of the ion exchange resin while producing water without adding additional chemicals such as acid and alkali, and having a continuous water production capacity.
What factors influence EDI (electro-deionization) module working?
Based on the analysis of the working principle of EDI desalination equipment, in the process of operation and use, the main factors affecting the use of EDI are feed water quality, pressure, flow rate, voltage, etc., which are discussed below.
Feed water quality to meet the requirements of EDI operation
The operation of the EDI module requires high-quality feed water (see the table below). Generally, in the pre-treatment of EDI, it is necessary to set up a reverse osmosis membrane module to pre-treat the raw water. The water that meets the standard after the two-stage RO treatment can generally be used as the influent of the EDI.
| Item | Data |
| Conductivity | <60μs/cm |
| Total exchangeable anions (Calculated as CaCO3) | ≤35mg/L |
| pH | 7.0~9.0 |
| Water temperature | 5~35°C |
| Total hardness (Calculated as CaCO3 ) | <1.0mg/L |
| Residual chlorine in influent | ≤0.02 mg/L |
| TOC | ≤0.2mg/L |
| Oxidizing agent (Cl2/O2) | Non-detectable |
| Variable metals (Fe and Mn) | Fe≤0.01 mg/L, Mn≤0.01 mg/L |
| SiO2 | ≤0.5 mg/L |
| Feed water pressure | ≤0.4MPa |
Conductivity is a comprehensive indicator of the total amount of ions in water, which cannot directly represent the quality of pure water. The main reason is that conductivity cannot truly reflect the content of weak electrolytes in water, especially carbon dioxide content. For example, the same conductivity of 10μS/cm reverse osmosis pure water, which may be 5ppm carbon dioxide content, may be 35ppm. If its content is too high, EDI cannot work correctly. In addition, there are differences in the size of different ions and the polarity of water, so there are significant differences in the ability of EDI to remove these ions. For these reasons, feedwater conductivity can only be used as a reference indicator, while TEA is a more accurate measure of feedwater quality.
Variations in water quality can also directly affect changes in EDI operating parameters, mainly in the form of linear voltage increases, which can cause irreparable damage to EDI modules in severe cases. Common influent water quality fluctuations are mostly temperature and pH.
- When total hardness is less than 0.1ppm, EDI works best in the PH range 8.0~9.0.
- The temperature has a direct effect on system pressure and water production resistance. A decrease in temperature will decrease water activity, i.e., the Brownian motion of ions in the water will be weakened, which is macroscopically expressed as an increase in water viscosity and an increase in system pressure. In general, the exchange capacity of the membrane also decreases as the temperature decreases and vice versa. However, when the temperature exceeds a specific value, the product water quality will gradually deteriorate. It is mainly because the exchange process of ions and resins and ion exchange membranes is weakened by ion activity. Therefore, when the feed water temperature is low, the voltage should be increased appropriately to increase the power of ion migration and ionize water molecules more effectively. Therefore, pressure variation and control are other critical factor in the proper operation of the EDI module.
The effect of pressure on EDI operation
In EDI operation, the maximum feed water pressure should not exceed 0.4MPa. Therefore, as the pressure increases and decreases, the use of the EDI effect is affected.
Too high feed water pressure will reduce the service life of EDI equipment because if the pressure is too high, it will lead to the EDI module holding pressure, and the module will be easily deformed, causing mechanical damage and affecting the service life of the equipment.
The EDI water production flow will be affected if the feed water pressure is too low. Because if it is too small, it will directly lead to a decrease in the product water of the freshwater chamber. In addition, it will also make the ions in the concentrated water chamber cannot be removed entirely, resulting in scaling and clogging of the filling resin in the concentrated water chamber, which will affect the effectiveness of the EDI module.
The pressure of EDI water production should be 0.03~0.05MPa higher than the pressure of the concentrated water chamber. Suppose the pressure of concentrated water is higher than that of fresh water, i.e., the pressure of the concentrated water chamber is higher than that of the freshwater chamber. In that case, the long-term operation will lead to the deformation of the EDI module and affect the quality of the product water.
The effect of flow rate on EDI operation
When EDI is in operation, the influent and product water flow rate will gradually decrease with the extension of the use time of the module. The reason is that after EDI has been in operation for a long time, there will be fouling and clogging in the resin filled in the EDI stack, causing the water production of the module to decrease.
When it is found that the freshwater production flow of EDI decreases, the module should be prepared for online chemical cleaning to restore its water production. EDI feedwater flow rate should be reasonable. Otherwise, it will increase the desalination load of the module, resulting in substandard water quality in the freshwater chamber. The influent flow of EDI-concentrated water should not be too large. The large flow rate has a good effect on the ion removal in the concentrated water chamber, but the pressure of the concentrated water chamber increases, which will cause the extrusion of the freshwater chamber and easily deform the module.
The EDI polar water inlet flow is relatively stable. The unique polar water inlet of EcoLan ensures the water flow of the EDI polar water chamber. It avoids the phenomenon that no water in the polar water chamber takes away the heat generated by the plate during EDI operation, resulting in dry burning and irreversible damage to the EDI module.
The effect of voltage on EDI operation
During the operation of EDI, the current remains unchanged, and the voltage increases with the increase in resistance. However, the external DC power supply voltage fluctuation to the EDI stack will affect the quality of the EDI product water.
Suppose the external DC power supply voltage becomes large. In this case, the quality of EDI product water remains unchanged. However, the amount of electrical energy consumption is large, which accelerates the electrode reaction of the EDI cathode and anode, causing rapid electrode corrosion.
Cathodic reduction reaction: H2O → H+ + OH–, 2H+ + 2e– → H2↑
Anodic oxidation reaction: H2O → H+ + OH–, 4OH– → O2↑ + 2H2O +4e– or 2Cl– → Cl2↑ + 2e–
Suppose the external DC supply voltage becomes lower. In this case, the EDI product water quality decreases because the resistance inside the EDI module remains unchanged, i.e., the resistance to ions through the anion or cation membrane remains unchanged. In contrast, the external DC voltage supplied decreases, and some ions in the concentrated brine cannot pass through the anion or cation membrane.
In the operation of EDI equipment, the accumulation of particle pollutants in the module scaling and blockage, resin failure, or bacterial growth blockage of the module will cause EDI to remove the ion resistance increases and the operating voltage increases. When the blockage reaches a certain level, the product water quality of EDI will drop significantly, the water production will decrease, and the voltage will rise to the limit.
When starting or stopping EDI equipment, if there is no water but electricity in the EDI module, it may cause a short circuit in the power supply.
During the operation of the EDI desalination system, only by fully understanding the effects of various factors on the operation of the EDI can we identify the causes of abnormal conditions that occur during operation and then deal with them. Therefore, given the above problems, EcoLan suggests the following precautions for the operating voltage of EDI.
- During the operation of EDI, we should keep the external power supply voltage stable. If the external power supply is unstable, the EDI equipment should be stopped to avoid damage.
- When starting or stopping EDI equipment, the EDI module must be watered first and then powered, or powered off first and then watered, to avoid short circuits and damage to the module.
- During the operation of the EDI, observe and record its flow, pressure, water resistance, differential pressure, and other parameters. For example, suppose the product water quality decreases, the voltage increases, or the pressure drop of water production increases. In this case, the EDI module should be cleaned with chemicals in time to avoid the irreparable consequences that overloading the EDI module may cause.
How to clean EDI (electro-deionization) system in water treatment?
According to the operating status of the EDI system, the cleaning EDI system adopts the method of acid washing – disinfection – alkaline washing to clean the EDI module.
We must clean the EDI system from the freshwater, concentrated, and polar water chambers. That is, the cleaning solution enters the EDI stack from the “Raw Water Inlet” and “Concentrated Water Inlet” cleaning ports and returns to the cleaning tank from the “Product Water,” “Concentrated Water Outlet,” and “Polar Water Outlet” ports.
Acid Wash: The cleaning water tank contains a 2.0% hydrochloric acid solution and circulates for 30 minutes.
Rinsing: After the pickling solution in the cleaning tank is released, the water in the tank is rinsed to neutral, and then the “clean inlet” and the return pipe to the return water are cleaned to neutral.
Disinfection: Cleaning water tank configuration 0.2% H2O2 solution (approximately 10kg of 25% concentration H2O2 solution) cycle 50 minutes.
Rinsing: After the disinfectant solution in the cleaning tank is released, clean the EDI to the backwater in the tank to the conductivity down to 100μs/cm or less.
Alkaline wash: The water tank is configured with 1.2% NaOH + 5.0% NaCl solution and circulates for 50 minutes.
With the continuous maturing of EDI equipment and pre-treatment processes, the quality of EDI product water is getting higher and higher. Moreover, since the beginning of the 21st century, the effluent resistivity of EDI is generally 15~18MΩ▪ cm (25ºC), which fully reaches the treatment level of traditional ion exchange technology, and the removal rate of weak electrolytes is better than that of conventional ion exchange method. Therefore, the EDI process is increasingly used to produce pure and ultrapure water.